Chemical Materials as Heritage: The Hafkenscheid Collection (ca. 1825) at Haarlem

Cet article a été élaboré par CultureSciences-Chimie et fait l'objet d'une co-publication.

Starting in Great Britain during the 1960s, the study of industrial heritage has increasingly been practised in many European countries. Special journals have been launched, including in 1976 the French journal L'Archéologie industrielle en France, renamed Patrimoine industriel in 2014. In the literature on industrial heritage, the attention paid to chemical heritage is almost neglectable. Among the numerous books published since 1970, only R. Angus Buchanan’s Industrial Archaeology in Britain (1972) devoted a separate chapter to the chemical industries. Another early pioneer was W. Alec Campbell, who in his book The Chemical Industry (1971), paid attention to old chemical sites and to chemical investigations of polluted soils and waste heaps of the British alkali industry ((R.A. Buchanan, Industrial Archaeology in Britain (Harmondsworth, Penguin, 1972); W. Alec Campbell, The Chemical Industry (London: Longman, 1971).)).

There is one great exception though, which is the special issue Le patrimoine industriel de la chimie ((Florence Hachez-Leroy (ed.), Le patrimoine industriel de la chimie. A special issue of Patrimoine Industriel – archéologie – technique – mémoire, no. 69 (Cilac: Paris, 2016).)). With almost twenty chapters on French chemical industries and sites, and more than five chapters on archives and collections, this book is unique in its kind. But even in that book there is not much attention paid to old collections of chemical substances. Those collections are dispersed over many different museums, and were seldom the subject of study. Therefore the investigation of the so-called ‘Hafkenscheid Collection’ of painting materials, presented in this paper, merits special attention ((For full details, see: Ineke Pey and Ernst Homburg, Een kabinet vol kleur: De collectie schildersmaterialen van de Amsterdamse verfhandelaar Michiel Hafkenscheid (1772-1846) (Nijmegen: Vantilt, 2018).)).
The Collection
The Hafkenscheid Collection includes more than 370 samples or materials of various kinds, such as pigments, gums, resins, adhesives, mordants, metal ores, chemicals and natural dyes, mainly used by artists and house painters, but also in dyeing and in other urban trades. The collection was created by the Amsterdam druggist and wholesaler of painting materials Michael Hafkenscheid (1772-1846) during the first decades of the nineteenth century. It probably served as a reference collection for his trading activities, but perhaps also to educate his eldest son and successor Antonius Hafkenscheid (1804-1877).

The collection was stored in a wooden cabinet, kept near at hand by the trading firm of M. Hafkenscheid & Zoon. When that firm was sold to the Ivormica company by the end of 1927, the cabinet and collection were sold to the Department of Inorganic Chemistry of Delft University of Technology. Some samples from the collection were used in pioneering scientific investigations of old paintings by the chemist and restorer Martin de Wild (1899-1969), who was one of the first in the Netherlands to initiate scientific research on paintings ((A. Martin de Wild, Het natuurwetenschappelijk onderzoek van schilderijen, PhD thesis, TH Delft, 12 June 1928 (printed The Hague, 1928). An English translation was published a year later under the title The Scientific Examination of Pictures (London: G. Bell & Sons, 1929).)).

Bringing the collection to life
For many decades the collection had almost been forgotten. It was stored in the basement of the chemical laboratory at Delft, was rarely used in research, and never investigated as such. This changed in 1983 when the chemical analyst and student of art history Ineke Pey, on the advice of a colleague from Leiden University, decided to investigate the collection in depth and write her Master Thesis on that topic.

During the following year she described the entire collection in detail, and employed a whole array of laboratory techniques in order to determine and verify the (chemical) composition of about 190 different mainly inorganic samples. Non-investigated samples were often other examples of the same product, or samples of dry and unprepared plant materials. On a list (inventory), probably dating back to the 1830s, the content of the entire collection had been written down by hand. All 190 samples were investigated with different techniques such as ordinary (light) or polarised-light microscopes, and with so-called ‘wet chemical microanalysis’ ((Microanalysis is the chemical identification and quantitative analysis of very small amounts of chemical substances (generally less than 10 mg). (Wikipedia). In practice, we used circa 5 small crystals for each test, which was well below the indicated threshold of 10 mg..
In most cases, these two methods already gave sufficient information to identify and characterise a sample. When more detailed information was required, more advanced instrumental techniques were used: infrared spectroscopy, laser micro-spectrum analysis, X-ray diffraction, scanning electron microscopy, high-pressure liquid chromatography (for prepared organic dyes), electron microprobe E.D.S. (energy dispersive spectroscopy, for dyes on substrates), flame-emission photometry, and, finally, atomic absorption spectroscopy. For experiments with more advanced equipment, great help was received from the art research laboratories of the Dutch and Belgian cultural heritage organisations, respectively in Amsterdam and Brussels ((Pey and Homburg, Een kabinet vol kleur, pp. 63-65, 274-279.)).
As indicated above, determination of chemical composition was in most cases performed by ‘wet chemical microanalysis’ ((Wet chemical analysis is chemical analysis in, usually aqueous, solution. In this aqueous solution containing the reagents (see the example below), we added the crystals, so that the identification reaction took place in solution.)), with the help of spot-tests ((A spot test is a simple and efficient technique where analytical assays are executed in only one, or a few drops, of a chemical solution, without using any sophisticated instrumentation. (Wikipedia))), in combination – if necessary – with (polarised-light) microscopy. These methods were chosen because we wanted to analyse only very small samples, in order to disturb the collection as little as possible. The chemical spot tests were performed under a stereo microscope, or dissecting microscope, which is an optical microscope designed for low magnification observation of a sample (in this case 80x), typically using light reflected from the surface of an object rather than transmitted through it. The chemical tests were done on small flat microscope slides ((We give here only a brief example, as an illustration, and do not intend to present a practical manual. For details of the method, see: Joyce Plesters, ‘Cross-Sections and Chemical Analysis of Paint Samples,’ Studies in Conservation 2 (3) (1956), 110-157, esp. pp. 127-129, 143-145; Fritz Feigl, and Vinzenz Anger, Spot tests in inorganic analysis, 6th rev. edition (Amsterdam ; New York: Elsevier, 1972); and De Wild, The scientific examination of pictures.)).
To make this more concrete for the reader, we will focus on the investigation of a red-orange pigment from the collection. On the hand-written inventory-list of the collection that sample was called true “orange minraal” from Paris. The name orange mineral refers to a purer kind of red lead, which is a lead oxide. Despite that indication on the inventory-list, chemical investigation was crucial for several reasons:
- to check whether the order of materials inside the cabinet still corresponded with the list after 150 years;
- to check whether the pigment indicated was perhaps mixed with other substances;
- to check whether the composition of pigments from the trade practices corresponded with the theoretical composition mentioned in handbooks.
In theory, if the order of the materials in the cabinet and the order on the list did not correspond (anymore), several other red and orange pigments on the list could be candidates for the sample investigated: iron oxides, such as red ochre (Fe2O3), red lead (Pb3O4), vermillion (HgS), realgar (As2S2), and organic lakes, such as madder lakes (madder dye on alumina).
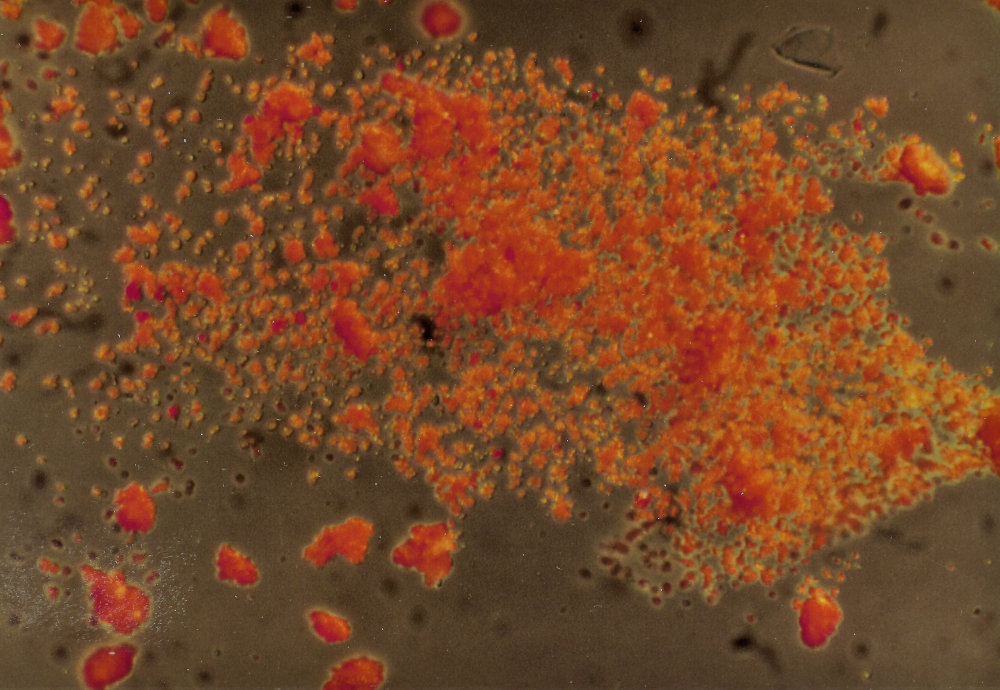
The sample was first investigated under a microscope, with a magnification of 200x, with incident and transmitted light. This showed us small bright orange crystals in many different shapes, which were mostly clear and transparent. Some of the orange crystals were opaque. In transmitted light there were dark particles with a grainy surface, with very few white and metallic black crystals in between the far greater number of orange ones. These observations made it already improbable that the sample was a usually very fine powdered madder lake.
After this visual inspection, the sample was investigated with chemical tests. Drops of test liquid, such as acids and bases, were added while the reaction was viewed under a stereo microscope. HCl, of a strength of 3 mole/ litre, decolorized the sample in the cold a bit slowly, but quickly after heating, leaving a white residue, and a few brown crystals. There was no emission of the ugly smelling H2S, which made the presence of vermillion and realgar very improbable. Of the pigments mentioned, only red lead (Pb3O4) dissolves in the HCl aqueous solution mentioned above, leading to a characteristic white precipitate of PbCl2 behind ((Pb3O4 + 4 HCl = 2 PbCl2 (white precipitate) + PbO2 (brownish) + 2 H2O. The equations mentioned here and below are tentative, because in the reactions between the crystals and drops of reagents the different reactants are not added in stoichiometric proportions. It is the characteristic colour of a resulting product that is crucial in these cases.)).

In order to check whether the pigment was indeed red lead, specific tests on Pb were done. Tests on Pb (II) with KI in HCl were positive, giving a characteristic precipitate of yellow PbI2 ((Pb3O4 + 4 HI = 2 PbI2 (yellow precipitate) + PbO2 (brownish) + 2 H2O.)). Also with K2CrO4 – in the presence of HCl – the test was positive, because a yellow precipitate of PbCrO4 resulted ((Pb3O4 + 2 K2CrO4 + 4 HCl = 2 PbCrO4 (yellow precipitate) + PbO2 (brownish) + 4 KCl + 2 H2O.)). In acetic acid the sample dissolved ((Pb3O4 + 8 HAc = 2 PbAc2 + Pb(OAc)4 + 4H2 (both in solution).)). In concentrated HNO3 the sample turned brown, which confirmed the formation of more lead dioxide (PbO2) ((Pb3O4 + 2 HNO3 = 3 PbO2 (brown precipitate) + 2 HNO2.)). The reaction product was dried, then dissolved in acetic acid. It showed a brown colour that was typical of the triple nitrite reaction, which confirms the presence of a double salt of three elements (in this case: PbNO2, PbNO2, and Pb(NO2)2). On the basis of these results we could conclude that the sample was indeed red lead or minium (Pb3O4), and that (a) the correspondence with the inventory-list was perfect; (b) the pigment was rather pure, and only mixed with small traces of dark contaminants (PbO2); and (c) the composition PbO2.2PbO of the red lead confirmed what was theoretically expected.
The results of these extensive experiments were documented in 1985 in a Master Thesis written by Ineke Pey. In that thesis, research in (old) books on the history of pigments and their nomenclature was reported, in which the other author of this paper, Ernst Homburg, was also involved ((.B.F. (Ineke) Pey, De collectie Hafkenscheid. Een verzameling schildersmaterialen uit de eerste helft van de negentiende eeuw, 2 Vols., Master Thesis, Katholieke Universiteit Nijmegen, May 1985.)). In the following years, Ineke Pey published several articles on the Hafkenscheid collection, which made this unique set of painting materials quite well-known among art historians, restorers, and scientists involved in research on paintings ((E.B.F. Pey, ‘De firma Michiel Hafkenscheid en Zoon. Een negentiende-eeuwse handel in schildermaterialen te Amsterdam’, KNOB. Bulletin van de Koninklijke Nederlandse Oudheidkundige Bond, 86 (1987), 49-70; E.B.F. Pey, ‘The Hafkenscheid Collection. A Collection of pigments and painting materials dating from the first half of the 19th century’, Maltechnik Restauro, 93 (1987), 23-33; E.B.F. Pey, ‘The organic pigments of the Hafkenscheid Collection’, Restauro, 95 (1989), 146-150; Ineke Pey, ‘The “sample book” of the Amsterdam paintware trader Michiel Hafkenscheid (1772-1846), from A(sphaltum) to Z(innober green)’, in: E. Hermens, A. Ouwerkerk, N. Costaras (eds.), Looking through Paintings. The Study of Painting Techniques and materials in support of Art Historical Research (Baarn/ London, 1998), pp. 465-500.)).
Through these publications the collection ‘came to life’ again. In 1995 Delft Technical University donated the collection to Teylers Museum at Haarlem, which was a far more appropriate location for these materials. An exhibition devoted to the collection was held in 1995. Since then, some other researchers investigated samples from the collection. In our view, Teylers Museum should take utmost care to make sure no unique sample from the collection disappears or gets contaminated, considering that a few examples that were still there in 1984 are now missing.
Dating the collection
Michael Hafkenscheid had been employed by the trading firm Tollens, Usellino & Comp. in Amsterdam since 1806, or perhaps even earlier. He later became a partner, and from around 1820, he continued the firm under his own name. In 1826, he was joined by his son, who also became a partner in the firm. The name was then changed to M. Hafkenscheid & Zoon.
Tollens, Usellino & Comp. was part of a network of firms owned by members of the Tollens family, located in Ghent, Rotterdam and Amsterdam. It included trading firms, shops with painting materials and small workshops for the manufacture of brushes or pigments ((Pey and Homburg, Een kabinet vol kleur, pp. 25-33.)).

As a result of the long history of the firm, it is understandable that there are also some samples from before 1806 in the collection. On the basis of written dates found on paper wrappers around some samples, as well as on the inventory from the 1830s, it appears that the oldest samples found in the collection were a green pigment from berries (eighteenth century), amber (1802), Dutch pink (1803), Naples yellow (1804) and caput mortuum (iron ochres) (1804). The newest samples mentioned in these written sources were rottenstone (1831) and turmeric (1832) ((Pey and Homburg, Een kabinet vol kleur, pp. 63-65.)).
There are also a few novel pigments in the collection, that had just been invented, such as chromate yellow (1815). But many other new pigments invented in the first half of the nineteenth century were missing. This confirms our conclusion that the collection was created between ca. 1800 and ca. 1835, without additions from a later date. This makes the collection a very interesting and rather unique example of material chemical heritage from the earliest decades of the nineteenth century. Because of its unique character the collection can be important for investigators working in different areas, such as art history, history of chemical technologies, and business history.
Relevance for the history of art
For about a century, chemical and other scientific investigations of paintings have played an ever-growing role in the history of art, and in the restoration of paintings in particular ((Geert Vanpaemel, ‘X-rays and old masters. The art of the scientific connoisseur,’ Endeavour 34 (2) (2010), 69-74.)). These investigations are essential to know more about earlier restorations of old paintings. What painting materials were originally employed? What pigments or varnishes were added later? What variety of a certain pigment is needed to restore a painting in the most adequate way? Questions such as these can only be answered by employing the most advanced scientific techniques possible.

But using these methods of analytical chemistry is not sufficient. With these techniques one can measure, for instance, the percentages of lead, copper, sulfur, or carbonates that a certain sample from a painting contains, and propose a chemical formula; but in order to give a correct interpretation in terms of the pigments used by the artist, knowledge of ancient artists materials is also needed ((See for instance: R.D. Harley, Artists’ Pigments c. 1600-1835: A Study in English Documentary Sources, 2nd ed. (London, etc.: Butterworth, 1982); Nicholas Eastaugh, et al., The Pigment Compendium: A Dictionary of Historical Pigments (Oxford: Elsevier Butterworth-Heinemenn, 2004).)). In theory, one could study handbooks for painters from the seventeenth to the nineteenth century to discover the names of the materials used, but that knowledge will not solve all the puzzles. Indeed, in the course of time, the composition of materials has changed, even if the name stayed the same, due to the replacement of natural materials by synthetic ones, or because of the evolution of manufacturing techniques, or due to the shift from one mine to another, etc. So for instance, white lead made in the seventeenth century differs from white lead manufactured today ((Ernst Homburg and Johan H. de Vlieger, ‘A victory of practice over science: The unsuccessful modernisation of the Dutch white lead industry (1780-1850)’, History and Technology 13 (1996), 33-52.)).
Seen in that light, the Hafkenscheid Collection dating from the years 1800-1835 – just before the great industrial revolution in pigment production – still contains many painting materials that were also used during the seventeenth and eighteenth centuries. Therefore, it can be of great relevance as a reference collection for the investigation and restoration of old paintings. Indeed, for those tasks, good collaboration within the ‘triangle’ of (1) art history, (2) laboratory investigations, and (3) knowledge of historical reference collections is crucial.
Relevance for the history of chemical technologies
As noted in the previous section, the chemical composition of painting materials has changed over time, even when the trade names of the products stayed the same. Although books on early chemical industries and technologies do exist, the description of production processes in those books is often rather general ((G. Fester, Die Entwicklung der chemischen Technik, bis zu den Anfängen der Grossindustrie; ein technologisch-historischer Versuch (Berlin: Julius Springer, 1923); Archibald Clow and Nan L. Clow, The Chemical Revolution: A Contribution to Social Technology (London: Batchworth Press, 1952).)). They are mostly based on books from the seventeenth to the nineteenth century that do not disclose many trade secrets that were employed in practice and therefore do not give reliable information on the composition of the product ((For a critical analysis in the case of pharmaceuticals, see for instance: G.Schröder Die pharmazeutisch-chemischen Produkte deutscher Apotheken im Zeitalter der Chemiatrie (Braunschweig 1957))).

Chemical analysis of samples from former centuries is therefore a valuable addition to our knowledge of the production processes taking place in pre-modern chemical industries. For instance, the impurities detected in the samples give information on the raw materials used, and on the actual production processes that were carried out. The chemical analysis of samples can also prove that two products with the same trade name might have a totally different chemical composition. The Dutch name ‘berggroen’ (mineral green; green verditer; green bice) can serve as an example. After our chemical analysis it appeared that the mineral malachite, as well as synthetic basic copper carbonates, and green chemicals with another composition were all traded under the name ‘berggroen’. The same is the case for products named ‘bergblauw’ (mineral blue), with could refer to both natural azurite and the synthetic Prussian blues, invented during the early eighteenth century. In order to clarify puzzles such as these, chemical analysis of samples from historical collections, such as the Hafkenscheid Collection, is crucial ((Pey and Homburg, Een kabinet vol kleur, pp. 197-199, 206.)).
Relevance for business history
The fact that the material samples in the Hafkenscheid Collection were accompanied by an inventory dating back to the early nineteenth century, on which some manufacturers were mentioned, and that in some cases samples were also packed in wrappers with names of suppliers, was a very lucky circumstance. Raw materials, for instance, came from Liège, Germany, Cologne, Kassel, Salzburg, France, England, Bristol, Spain, Malaga, Sweden, St. Petersburg, Italy, Venice, Verona, the Levant, Smyrna, Aleppo, Turkey, Bengal, the East-Indies, China, Brazil, America, and many more places, which gives us information on the very wide trade-network of the port of Amsterdam that was utilized by Michael Hafkenscheid ((Pey and Homburg, Een kabinet vol kleur, pp. 258-273.)).

In some cases, specific manufacturers were also mentioned. Examples are white leads from a factory in Hull, from Kremnitz in Austria, and from Dutch factories in Bodegraven, Schiedam, Rotterdam and Wormerveer; or Frisian greens from Loensma in Buiksloot and Suringar & Nauta in Leeuwarden; or Bremer green from Hafkenscheid’s own factory. Examples such as these give us an insight into the specific trade relations of the company. They supplement information on industry from published and archival sources, and, finally, in combination with chemical analysis, they can provide information on differences in the quality of the products on the market.
Finally, the ambiguities in the nomenclature of trade products can supply information about the different markets for painting materials. The fact, for instance, that Prussian blue was traded as ‘mineral blue’, the traditional name of azurite, is an indication that when Prussian blue was invented, and not yet very known, it was put on the market in competition with the traditional natural product. Only later, in the nineteenth century, when it was more established and when chemical analysis had improved, was it more often traded under its specific name ((On the history of Prussian blue, see for instance: Alexamder Kraft, ‘What a chemistry student should know about the history of Prussian blue,’ ChemTexts 4, 16 (2018). https://doi.org/10.1007/s40828-018-0071-2.)).

Conclusion
We hope to have demonstrated that the Hafkenscheid Collection is unique and very broad (370 samples), dating back to the years ca. 1800 – ca. 1835, and that the study of the collection has a great relevance for fields as different as art history, history of chemical technology, and business history.
Notes
Pour citer cette ressource :
Ernst Homburg, Ineke Pey, Chemical Materials as Heritage: The Hafkenscheid Collection (ca. 1825) at Haarlem, La Clé des Langues [en ligne], Lyon, ENS de LYON/DGESCO (ISSN 2107-7029), octobre 2020. Consulté le 10/02/2026. URL: https://cle.ens-lyon.fr/anglais/se-former/dnl/dnl-chimie/chemical-materials-as-heritage-the-hafkenscheid-collection-ca-1825-at-haarlem


